ISO 17025 Certification
S & J Auditing and Consulting is committed to guiding your laboratory to successfully achieve ISO 17025 Certification, ensuring adherence to the highest standards in calibration and testing.
Fast and Efficient Process
Highly Experienced Consultants
Servicing All Industries
Certification Guaranteed
ISO 17025 Accreditation
The Benchmark for Laboratory Excellence
ISO 17025 is a globally recognised standard for calibration and test laboratories. It is one of ISO’s most trusted sets of requirements, ensuring that laboratories can consistently produce reliable and accurate results. This ISO 17025 standard is integral in determining and representing uncertainty, and in ensuring that certificates of test results or calibration are traceable to national or international standards, which is crucial for lab accreditation.
Clause 1 – Scope
Clause 2 – Normative References
Clause 3 – Terms and Definitions
Clause 4 – General Requirements
Clause 1 outlines the essential requirements of the ISO 17025 standard for the competence, impartiality, and consistent operation of laboratories involved in testing, calibration, and sampling. It applies universally to all laboratories, regardless of size or scope, and encompasses testing, calibration, and sampling activities using standardised, non-standardised, and internally developed methods.
Clause 2 specifies the key documents required for the correct application of ISO 17025. These references provide additional guidelines, rules, or characteristics for various activities or their outcomes, ensuring consistency and adherence to recognised standards. Importantly, the sole normative reference in ISO 17025:2017 is ISO/IEC Guide 99, which is the International Vocabulary of Metrology (VIM) – Basic and general concepts and associated terms
Clause 3 defines specific technical and general terms relevant to the standard, ensuring a consistent understanding among all users. This uniformity is crucial for clear communication and compliance. The clause refers to terms and definitions found in ISO/IEC Guide 99 (VIM) and ISO/IEC 17000.
Clause 4 of the ISO 17025 requirements emphasises the need for impartiality in all laboratory operations, underscoring the importance of delivering unbiased results. It also covers maintaining the confidentiality of customer information, except where disclosure is required by law. Laboratories must be accountable for all their activities, continuously identifying and managing risks to impartiality.
Clause 5 – Structural Requirements
Clause 6 – Resource Requirements
Clause 7 – Process Requirements
Clause 8 – Management System Requirements
Clause 5 details the organisational structure necessary for effective laboratory operations. Laboratories must clearly define and document the roles and responsibilities of management and key personnel involved in testing and calibration activities. Additionally, laboratories should document their scope of activities, including any conformity assessments they do not perform, and meet specific requirements to ensure independence and impartiality if they are part of a larger organisation.
Clause 6 specifies the requirements for human resources, the physical environment of the laboratory, specialised equipment, and measurement traceability. It also covers the management of products and services provided by external suppliers, ensuring all necessary resources are maintained to support reliable testing and calibration.
Clause 7 outlines the protocols for handling inquiries, requests, and contracts, as well as selecting and validating methods used in testing and calibration. It addresses procedures for sampling, managing test items, and ensuring the quality of laboratory outputs to guarantee reliable and accurate results.
Clause 8 provides laboratories with the option of implementing either a comprehensive management system aligned with ISO 9001 or a simpler system tailored specifically to testing and calibration activities. Regardless of the system chosen, it must effectively support and demonstrate consistent fulfilment of the requirements in Clauses 4 to 7, focusing on continuous improvement and operational efficiency to maintain lab accreditation.
Key Benefits of Implementing ISO 17025
Adopting ISO 17025 offers significant advantages:
Assured Quality and Reliability
Guarantees the quality of laboratory practices and the reliability of results.
Global Data Consistency
Meets the growing demand for globally consistent and credible data.
Enhanced Reputation
Boosts both international and domestic recognition of the laboratory.
Technical Competency
Establishes technical competency in case of legal or legitimacy issues.
Reliable Test Results
Increases the reliability of test outcomes produced by the laboratory.
Our 10-Step Process to ISO 17025 Certification in Australia
Our client-focused approach guarantees a thorough and effective ISO 17025 system development, guiding you through the 10-step process
Tailor your documentation to embody your distinctive brand and operational approach, ensuring coherence with your organisational ethos.
Our ISO 17025 consultants will perform a comprehensive analysis of your company’s risks and opportunities to craft a bespoke ISO management framework.
Formulate processes, policies, and procedures explicitly designed to fulfil your business objectives and ISO 17025 requirements.
Our specialists will collaborate closely with you at each stage, guaranteeing your utmost satisfaction throughout the journey.
Deliver in-depth training to your team, empowering them to execute the new protocols proficiently.
Implement the ISO management systems and conduct meticulous assessments to confirm adherence to ISO 17025 standards.
Pinpoint and rectify any essential corrective measures to secure full compliance before ISO 17025 certification.
Coordinate all facets of the ISO 17025 certification audits, facilitating a smooth and efficient evaluation process.
Offer expert counsel during the audits, supporting your pursuit of successful ISO 17025 accreditation.
Devise a strategy for continual upkeep and enhancement of your ISO 17025 system, fostering ongoing improvement and enduring compliance.
Testimonials
The process of ISO9001 certification was much easier than we anticipated due to engaging Scott as our consultant. Scott was fantastic to work with – he was confident, direct and focused on getting the job done. We interviewed a number of consultants before choosing Scott and he made us feel comfortable before and during the process. Scott did exactly what he said he would; he helped us through the entire process and ensured the audit ran smoothly. To top it all off, we gained certification over 1 month earlier than our deadline – a welcome bonus.

Haylee Evans
Operations and Financial AnalystWe engaged Scott to assist with the development of our safety, quality and environmental management systems from the ground up and through to full ISO certification. Scott’s approach was refreshing and the system we built together is both efficient and has significantly improved our daily practices. We look forward to continuing a long working relationship with Scott and S&J Auditing and Consulting.

Matt Lucarelli
Owner - Capstone ConstructionScott Smith at S&J Auditing and Consulting have implemented our HSEQ system here at Robowash which has helped our business immensely. Not only has the system allowed us to win and maintain contracts with pre-qualification being simplified, but it has given us a greater understanding of our system and processes allowing us to improve our business.

Jay Jasper
Managing DirectorS&J Auditing and Consulting have made it very easy for Mentis to obtain their ISO accreditation. Through Scott’s knowledge of the standards and his ability to understand our business, Scott was able to develop a quality system that suited Mentis in a timely manner and within budget. Mentis have also engaged SJ Auditing and Consulting to maintain the ISO system on a regular basis, this has helped Mentis become more efficient, by allowing staff to focus on their core tasks and still be connected to the quality process.

Dale Drazevich
Australian Managing Director
Over 70 5-star Google Reviews makes us the best choice for achieving ISO 17025 Certification.
Understanding ISO/IEC 17025:2017
ISO/IEC 17025:2017 specifies general requirements for the competence, impartiality, and consistent operation of laboratories. It is applicable to all organisations performing laboratory activities, regardless of size, and is widely used by laboratory customers, regulatory authorities, accreditation bodies, and others to confirm or recognize the competence of laboratories.
ISO 17025 - Lab Accreditation Certificate
Laboratories conforming to ISO 17025 will generally operate in accordance with the principles of ISO 9001. This standard requires laboratories to plan and implement actions to address risks and opportunities, thereby enhancing the effectiveness of the management system, achieving improved results, and preventing adverse effects. Adhering to ISO 17025 requirements ensures that laboratories maintain high standards of quality and reliability.
ISO 17025 Certification: A Path to Excellence
ISO 17025 certification is not just a compliance measure; it is a testament to a laboratory’s commitment to excellence in testing and calibration. By achieving this certification, your laboratory demonstrates a high level of technical competency and reliability. With our ISO 17025 accreditation consultants, we will be able to help achieve your certification goals and uphold the highest standards in your industry.
Open new doors with ISO 17025 certification
it's ok , we don't like spam either!
The Evolution of ISO/IEC 17025: Revisions & Updates
Ever wondered why ISO/IEC 17025 seems to get a "makeover" every so often? Just like your favorite tech gadget, this standard is regularly updated to keep pace with the latest technological and market shifts.
2005 Revision
Setting the Stage for Modernization
- Modern Alignment with quality management principles
- Enhanced Clarity in documentation
- Improved Testing Methods
2017 Revision
Embracing the Future
- Integration of modern IT techniques
- Structured risk assessment implementation
- Adaptation to market demands
Why This Matters to You
Deeper Insight
Understanding the historical context provides a richer appreciation of the current standard's rigor and relevance.
Enhanced Trust
By demonstrating the evolution, you position your organization as a forward-thinking leader in quality and compliance.
Future-Proofing
Insights into revisions can help labs and testing facilities prepare for upcoming changes, ensuring continual improvement.
Who Should Care About ISO/IEC 17025?
Not every standard is one-size-fits-all—but ISO/IEC 17025 isn't just for the lab geeks (although we love them!). It's designed for a diverse array of organizations and professionals who rely on precision, accuracy, and quality.
Calibration Laboratories
What They Do: Ensure that instruments and equipment are measured accurately.
Why It Matters: Accreditation under ISO/IEC 17025 boosts credibility and trust in their measurement capabilities.
Testing Laboratories
What They Do: Conduct critical tests for product safety, quality, and regulatory compliance.
Why It Matters: Adhering to ISO/IEC 17025 ensures results that are both accurate and reliable.
Forensic Institutions
What They Do: Provide scientific evidence for legal proceedings.
Why It Matters: Precision and standardized procedures can make all the difference in court.
Universities & Research Centers
What They Do: Advance scientific knowledge and support innovation.
Why It Matters: Aligning with ISO/IEC 17025 helps maintain rigorous research standards.
Accreditation Bodies
What They Do: Evaluate and certify the competence of labs.
Why It Matters: A robust standard simplifies the accreditation process and enhances transparency.
Other Sectors
Conformity Assessment Bodies, Product Certifiers, and Reference Material Producers can all benefit from the standard's structured approach to quality and risk management.
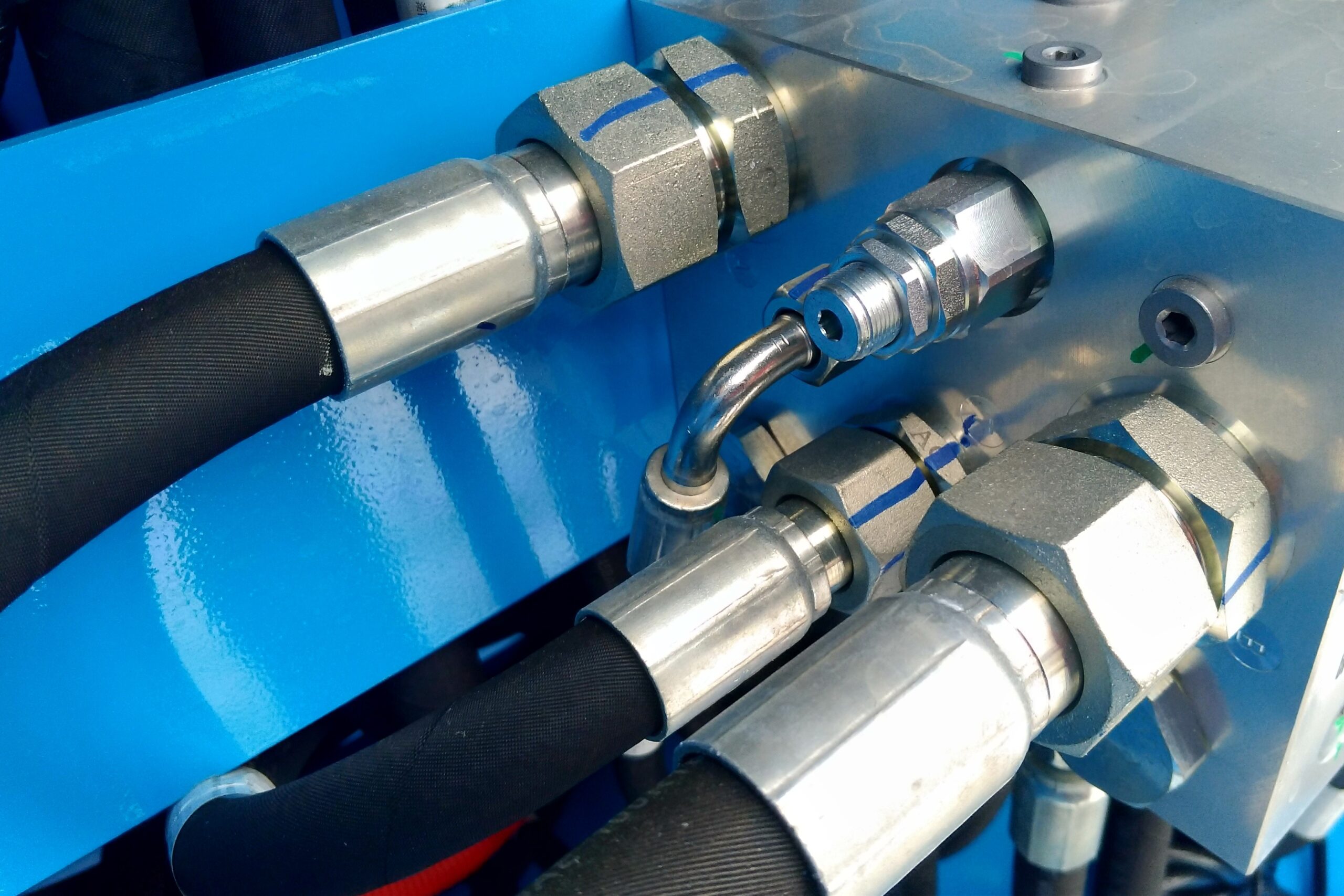
Industries we serve with our ISO 17025 consultancy services & more
Our expertise spans manufacturing, construction, electrical services, and hydraulic industries. Whether you're seeking initial ISO 17025 certification or maintaining your lab accreditation, our consultants provide tailored solutions to meet ISO 17025 requirements. We understand the unique challenges of various industries and can guide your laboratory through implementing and maintaining this crucial standard. View our list of industries below to see how we can support you on your journey.




















Learn more about out other certifications

ISO 9001
Quality Management Systems

ISO 14001
Environmental Management Systems

ISO 45001
Occupational Health & Safety Management Systems

ISO/IEC 17024:2012
General Requirements for Bodies Operating Certification of Persons

ISO/IEC 17065:2012
Requirements for Bodies Certifying Products, Processes & Services

ISO/IEC 17034:2016
General Requirements for The Competence of Reference Material Producers

AS9100
Aerospace Quality Management Systems
Start Your ISO 17025 Certification Journey
Begin your journey to ISO 17025:2017 certification with S & J Auditing and Consulting. Contact us to discover how we can help your laboratory achieve and sustain this critical certification.
Featured Case Studies
Read our case studies from some of WA & Australia’s most reputable companies.
Proudly trusted by







































Frequently Asked Questions For ISO 17025
What is ISO 17025?
ISO 17025 is the international standard for the competence of testing and calibration laboratories. This globally recognised standard sets the requirements for quality management systems and technical competence necessary for labs to deliver technically valid test results and calibrations.
What are the requirements for ISO 17025?
The requirements for ISO 17025 include a documented quality management system, technical competence, valid and repeatable testing or calibration methods, appropriate handling of test items, and quality assurance of test and calibration data.
What is the latest version of ISO 17025?
The latest version of ISO 17025 was published in 2017. It includes updates to accommodate changes in laboratory environment and technology.
What is the purpose of ISO 17025?
The purpose of ISO 17025 is to establish a global standard for testing and calibration laboratories regarding their capability to produce accurate and reliable results, thereby promoting confidence in their work both nationally and around the world.

































































